58 Minerals (A – Z)
(a slowly growing, but yet very incomplete and subjective selection, last update 12.04.2021)
You have the possibility to visualize the crystals structure of all these minerals in 3D not only with VESTA / Mercury but also online:
https://cds.dl.ac.uk/crystalworks/examples_minerals.html
Abelsonite
- Discovered only in 1969 (by the way the year of birth of the author of this blog) by Lawrence C. Trudell while he was exploring the Green River Formation (Utah, USA) for an oil shale project
- Named after Philip H. Abelson (1913–2004), a long-time editor of the journal Science, for his pioneering work in organic geochemistry
- Abelsonite is formed as a secondary mineral (and is one of the few organic minerals) on oil shale surfaces by conversion of chlorophyll – therefore, it is called a chemofossil (a fossil that consists only of chemicals remaining from the decomposition of a living organism)
- Abelsonite is the only known naturally occurring crystalline porphyrin derivative
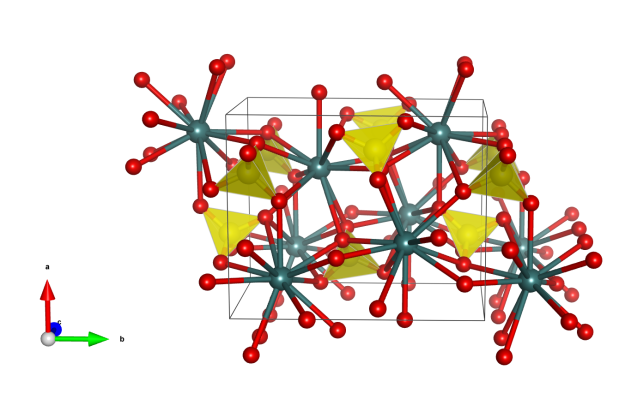
- Formula: NiC31H34N4
- Space group: P-1 (No. 2)
- Crystal system: triclinic
- Crystal class: -1
- Lattice parameters: a = 8.4416 Å, b = 10.8919 Å, c = 7.2749, α = 90.465°, β =113.158°, γ = 78.080°

Picture: CC BY-SA 3.0 Thomas Witzke – http://tw.strahlen.org/fotoatlas1/abelsonite_foto.html
Crystal structure (click on the picture to download the CIF):
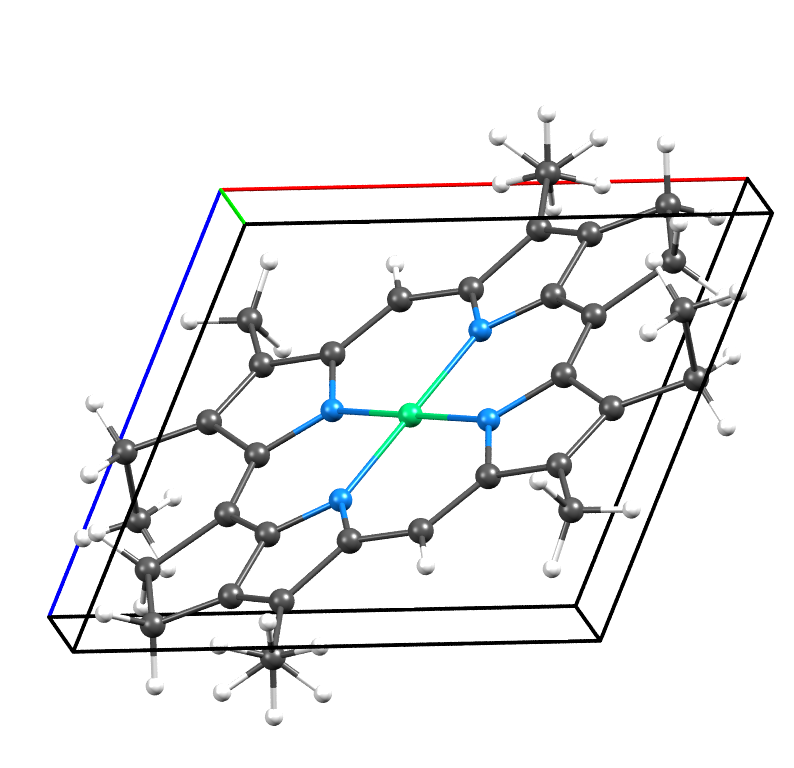
- Ni: green, N: blue, C: gray, H: white
- Note: Although a single Ni porphyrin molecule does not possess -1 symmetry, matching ethyl groups at roughly opposite ends of the molecule enable orientational disorder, in which molecules can randomly adopt one of two different orientations while still stacking in the same manner. The aggregate of these two random orientations produces an overall symmetry of P-1.
- Reference: Crystal structure of abelsonite, the only known crystalline geoporphyrin
Daniel R. Hummer, Bruce C. Noll, Robert M. Hazen, Robert T. Downs
American Mineralogist (2017) 102 (5): 1129-1132.
https://doi.org/10.2138/am-2017-5927
Adamite
- Named after the French mineralogist Gilbert-Joseph Adam (1795 – 1881)
- Typically occuring in weathered zones above zink ores
- Formula: Zn2(OH)(AsO4)
- Space group: Pnnm (No. 58)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 8.30 Å, b = 8.51 Å, c = 6.04, α = β = γ = 90°

Picture: Rob Lavinsky, iRocks.com – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
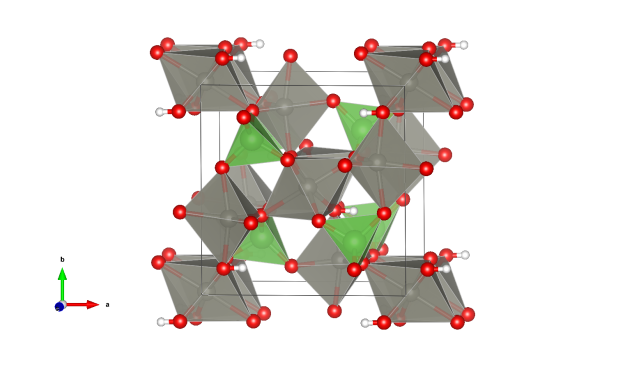
- AsO4 tetrahedra (green)
- ZnO6 octahedra (gray)
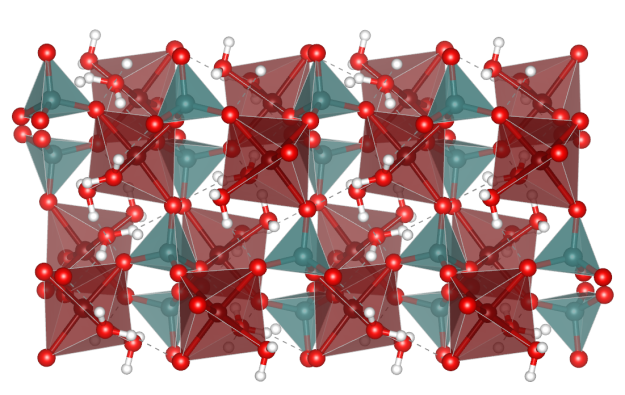
Afwillite
- Named after the abbreviated discoverer Alpheus Fuller Williams (1874–1953), CEO of De Beers Consolidated Mines at that time
- Formula: Ca3(SiO3OH)2 · 2 H2O
- It belongs to the nesosilicates, i.e. there are only isolated SiO4 tetrahedra
- Space group: Cc (No. 9)
- Crystal system: monoclinic
- Crystal class: m
- Lattice parameters: a = 16.278 Å, b = 5.6321 Å, c = 13.236, α = γ = 90°, β = 134.898°
Picture: – http://www.mindat.org/photo-356015.html | CC BY-SA 3.0
Crystal structure (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
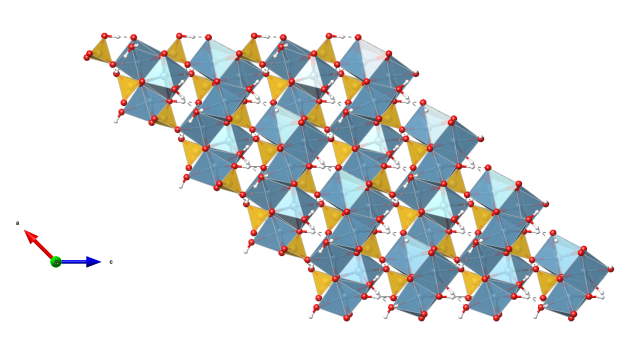
- Oxygen (red)
- Hydrogen (white)
- SiO4 tetrahedra (yellow)
- CaO7 polyhedra (light blue)
For a 3D interactive version, see here:
Anglesite
- Named after the type locality (a locality, where a particular mineral was first found/identified), which is here the Isle of Anglesey (Wales)
- Occurs as an oxidation product of primary lead sulfide (PbS, Galena) ore
- Formula: PbSO4
- Space group: Pnma (No. 62)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 8.48 Å, b = 5.40 Å, c = 6.96, α = β = γ = 90°

Picture: Rob Lavinsky, iRocks.com – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
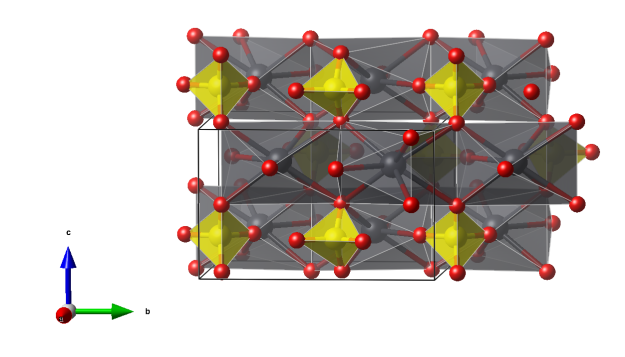
- SO4 tetrahedra (yellow)
- PbO8 irregular polyhedra (gray)
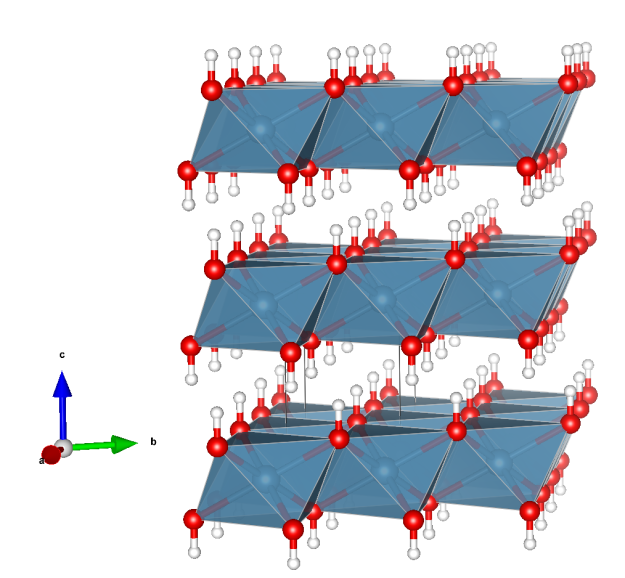
Antarcticite
- named after the type locality – the Don Juan Pond in Antarctica
- Antarcticite is very hygroscopic and is formed only in very arid regions and precipitates only from highly saline brines
- Formula: CaCl2 · 6 H2O
- Space group: P321
- Crystal system: trigonal
- Crystal class: 32
- Lattice parameters: a = b = 7.8759(2) Å, c = 3.9545(2) Å, α = β = 90°, γ = 120°
Crystal structure (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
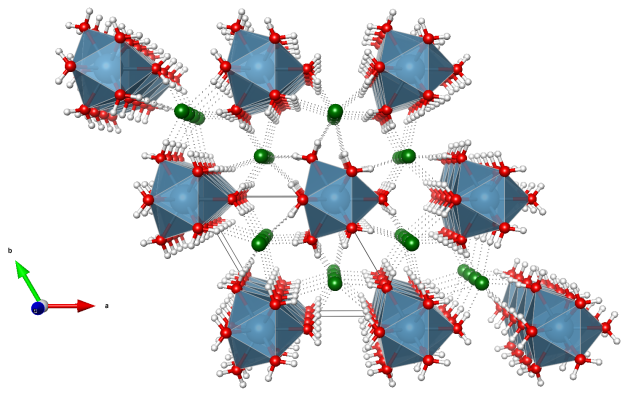
- every Ca ion is surrounded by nine water molecules
- the CaO9 polyhedra (blue) are face-connected and stacked along the c axis
- these faces are made up of three water molecules acting as bridging ligands between two Ca ions
- every Cl anion is involved in 6 H-bonds (dotted lines)
- Oxygen (red)
- Chlorine (green)
- Hydrogen (white)
For a 3D interactive version, see here:
Refs:
[1] T. Torii, J. Ossaka, Science 1965, 149, 975-977.
DOI: 10.1126/science.149.3687.975
[2] P. A. Agron, W. R. Busing, Acta Crystallogr. C 1986, 42, 141-143.
DOI: 10.1107/S0108270186097007
Aragonite
- named after the type locality Aragon (Spain)
- one of the two naturally occuring forms of calcium carbonate (the other being calcite)
- Formula: CaCO3
- Space group: Pmcn (No. 62)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 4.95 Å, b = 7.96 Å, c = 5.74, α = β = γ = 90°

Picture: Rob Lavinsky, iRocks.com – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
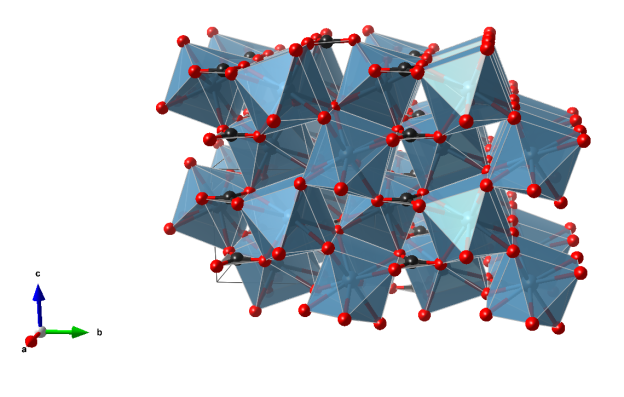
- CO3 triangles (black)
- CaO9 irregular polyhedra (blue)
Atacamite
- Named after its type locality, the Atacama desert in Chile.
- Atacamite is isostructural with Hibbingite [Fe2Cl(OH)3], and Kempite [Mn2Cl(OH)3]
- In 2002 it was found out that the jaws of the marine bloodworm Glycera dibranchiata contain Atacamite.[1]
- Atacamite is polymorphous with Botallackite and Clinoatacamite (both monoclinic).
- Formula: Cu2Cl(OH)3
- Space group: Pnma (No. 62)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 6.030(2) Å, b = 6.865(2) Å, c = 9.120(2) Å, α = β = γ = 90°

Picture: By Stefan Schorn – CC BY-SA 3.0
http://www.mineralienatlas.de/lexikon/index.php/Bildanzeige?pict=1081079762,
https://commons.wikimedia.org/w/index.php?curid=399210
Crystal structure[2] (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
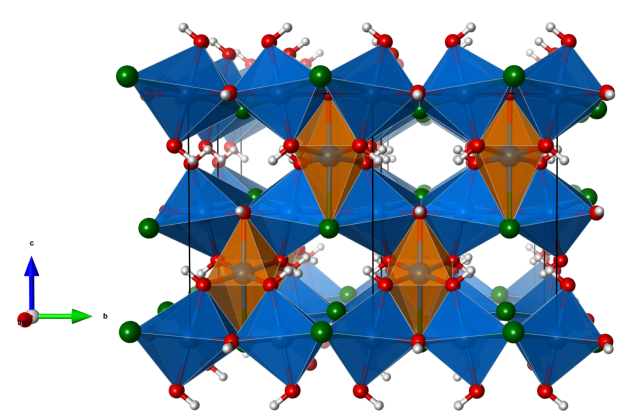
- CuO5Cl distorted octahedra (orange)
- CuO4Cl2 distorted octahedra (blue)
- Oxygen (red)
- Chlorine (green)
- Hydrogen (white)
For a 3D interactive version, see here:
References:
[1] H.C. Lichtenegger, Th. Schöberl, M.H. Bartl, H. Waite, G.D. Stucky
Science 2002, 298, 389-392.
DOI: 10.1126/science.1075433
[2] J.B. Parise, B.G. Hyde
Acta Cryst. C 1986, 42, 1277-1280.
DOI: 10.1107/S0108270186092570
Azurite
- named after the color azure, a variation of blue that is often
described as the color of the sky - one of the two basic copper(II) carbonate minerals, the other being malachite
- Formula: Cu3(CO3)2(OH)2
- Space group: P21/c (No. 14)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 5.01 Å, b = 5.58 Å, c = 10.35 Å, α = 90°, β = 92.4°, γ = 90°

Picture: Rob Lavinsky, iRocks.com – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
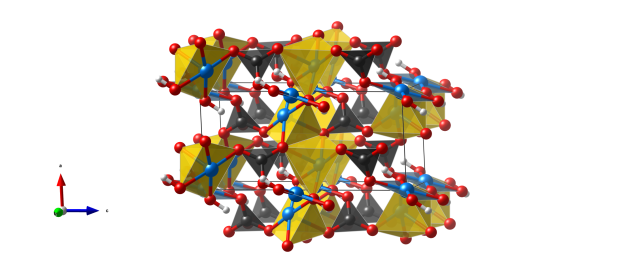
- CO3 triangles (black)
- CuO5 tetragonal pyramids (yellow)
Baryte
- named after the greek word for heavy (the density is relatively high
with 4.5 g/cm3) - Formula: BaSO4
- Space group: Pnma (No. 62)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 8.88 Å, b = 5.46 Å, c = 7.16 Å, α = β = γ = 90°

Picture: Carlesmillan – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
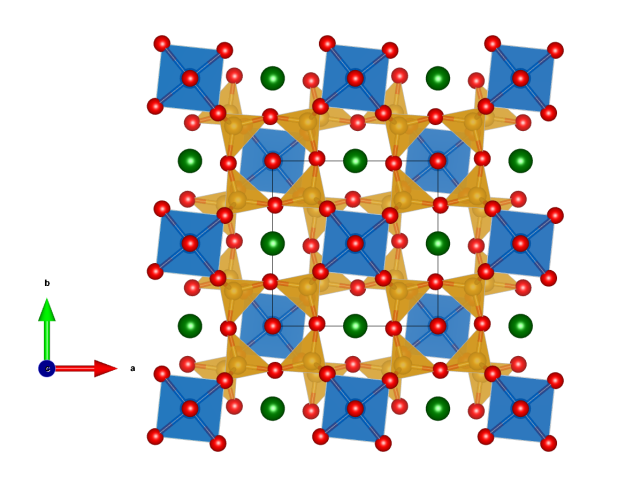
- slightly distorted SO42- tetrahedra (yellow)
- Ba2+ ions (green), which are surrounded by 12 oxygen atoms
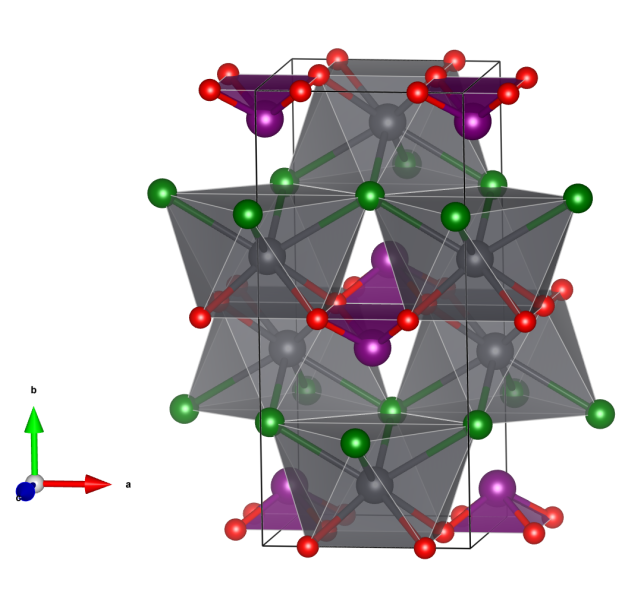
Bavsiite
- Named for the constituting elements Barium, Vanadium and Silicon.
- Bavsiite is polymorphic to Suzukiite, BaVSi2O7, which is orthorhombic.
- Formula: Ba2V2O2[Si4O12]
- Space group: I4/m (No. 87)
- Crystal system: tetragonal
- Crystal class: 4/m
- Lattice parameters: a = b = 7.043(1) Å, c = 11.444(2) Å, α = β = γ = 90°
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
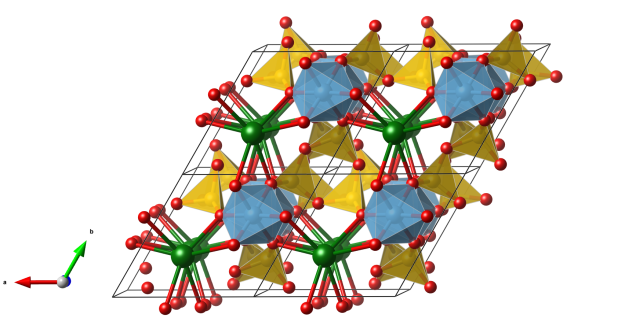
- BaO12 polyhedra (not shown as polyhedra, Ba green)
- SiO4 tetraedra (yellow) building single four-rings
- VO5 square pyramids (blue)
- Oxygen (red)
For a 3D interactive version on sketchfab, see here:
Reference:
Bavsiite, Ba2V2O2[Si4O12], mineral data and crystal structure
H.-P. Bojar, F. Walter, J. Baumgartner
Mineralogical Magazine 2019, 83, 821-827
DOI: 10.1180/mgm.2019.59
Bayldonite
- named after its discoverer, the British physicist John Bayldon
- Formula: PbCu3(AsO4)2(OH)2
- Space group: C2/c (No. 15)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 10.15 Å, b = 5.89 Å, c = 14.08 Å, β = 106.1°

Picture: Rob Lavinsky, iRocks.com – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
- Cu: blue, As: green, Pb: black, O: red, H: white
- elongated CuO6 octahedra
- AsO4 tetrahedra
- Pb surrounded by 7 oxygen atoms
Benitoite
- named after its type locality San Benito County (California)
- it is the official state gem of California
- Formula: BaTiSi3O9
- Space group: P-6c2 (No. 188)
- Crystal system: hexagonal
- Crystal class: -6m2
- Lattice parameters: a = b = 6.641 Å, c = 9.7579 Å, α = β = 90°, γ = 120°

Picture: Didier Descouens – CC BY-SA 4.0
Crystal structure (click on the picture to download the VESTA file):
- Ba: green, Ti: lightblue, Si: orange, O: red
- TiO6 octahedra, SiO4 tetrahedra
- three corner-connected SiO4 tetrahedra build a ring
Beryl
- named after the latin word beryllus or greek word beryllos, respectively, which is referred to “precious blue-green color-of-sea water stone”
- the first lenses were made of beryl, as glass could not be made clear enough; this is the origin of the German word for glasses, i.e. “Brille”
- there are important varieties, namely the blue Aquamarine (Fe2+ impurities), the Green Emerald (Cr3+ impurities), the Golden Beryl (Fe3+ impurities), the Pink or Rose Beryl named Morganite (after the financier J.P. Morgan, containing Mn2+ impurities), and the Red beryl
- Formula: Al2Be3Si6O18
- Space group: P6/mmc (No. 192)
- Crystal system: hexagonal
- Crystal class: 6/mmm
- Lattice parameters: a = b = 9.219 Å, c = 9.198 Å, α = β = 90°, γ = 120°

Picture: Aquamarine, Rob Lavinsky, iRocks.com – CC BY-SA 3.0

Picture: Aquamarine, Rob Lavinsky, iRocks.com – CC BY-SA 3.0

Picture: Golden Beryl, Rob Lavinsky, iRocks.com – CC BY-SA 3.0

Picture: Morganit, Rob Lavinsky, iRocks.com – CC BY-SA 3.0

Picture: Red Beryl, Rob Lavinsky, iRocks.com – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
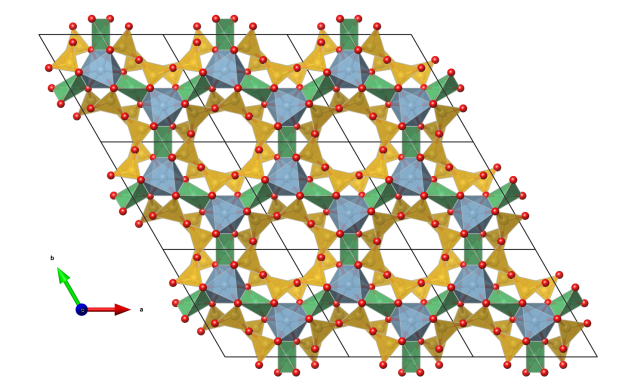
- yellow: SiO4 tetrahedra, six tetrahedra are corner-connected to form a ring
- green: distorted BeO4 tetrahedra
- blue: slightly distorted AlO6 octahedra
Blödite (also Bloedite)
- named after the German mineralogist and chemist Karl August Blöde (1773 – 1820)
- fun fact: the German adjective “blöd(e)” means “stupid”
- Formula: Na2Mg(SO4)2 · 4 H2O
- Space group: P21/a
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 11.126(2), b = 8.242(1) Å, c = 5.539(1) Å, α = 90°, β = 100.84(1)°,
γ = 90°
Crystal structure (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- SO4 tetrahedra (yellow)
- (distorted) NaO6 octahedra (purple)
- MgO6 octahedra (green)
- Oxygen (red)
- Hydrogen (white)
For a 3D interactive version, see here:
Refs:
[1] F. C. Hawthorne, The Canadian Mineralogist 1985, 23, 669-674.
(PDF)
Bornite
- named after the Austrian mineralogist Ignaz von Born
- also known as peacock ore because in air this mineral forms very quickly an iridescent coverage on its surface
- Formula: Cu5FeS4
- Space group: Pbca (No. 61)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 10.950 Å, b = 21.862 Å, c = 10.950, α = β = γ = 90°

Picture: Rob Lavinsky, iRocks.com – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
- yellow: Sulfur, blue: Copper, orange: Iron
- The structure is based on a cubic-closest packing of sulfide ions; the copper and iron ions are located in 3/4 of the tetrahedral voids of the packing. At temperatures above 228 °C the cations are completely randomly distributed over these sites, forming a genuine cubic phase, but in the low temperature modification the cations are more ordered, which is accompanied with a symmetry reduction to the orthorhombic crystal system
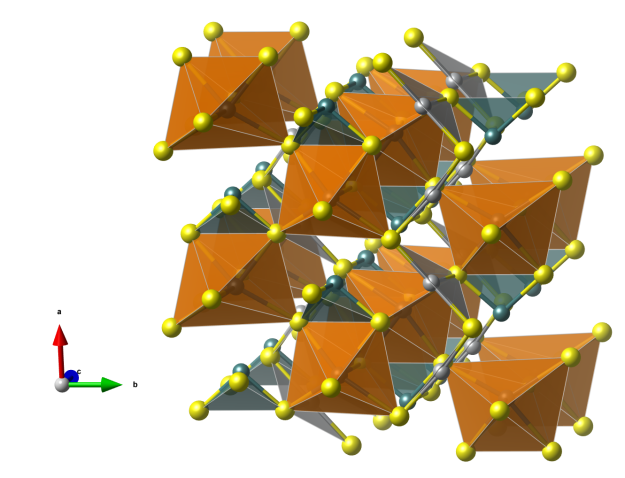
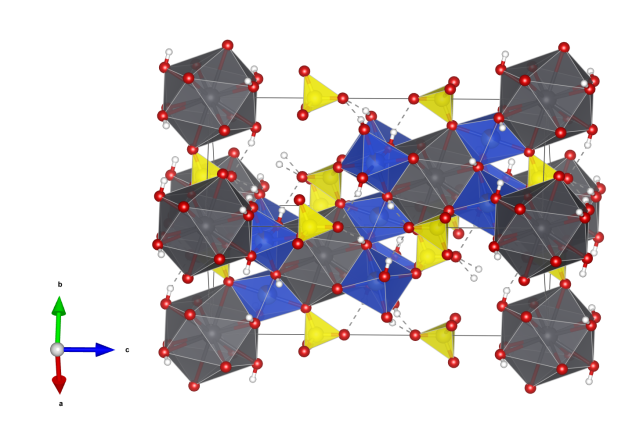
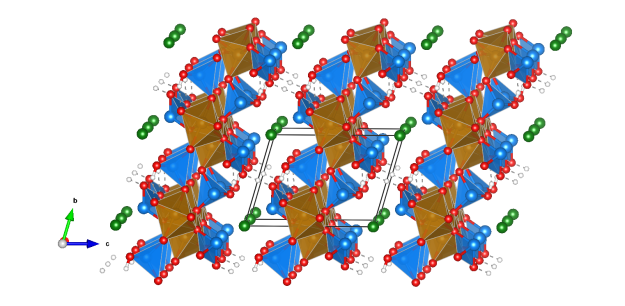
Botallackite
- Named in 1865 by Arthur Herbert Church for its occurrence in the Botallack Mine, Cornwall, England.
- Botallackite is polymorphous with Atacamite and Clinoatacamite.
- Formula: Cu2Cl(OH)3
- Space group: P21/m (No. 11)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 5.717(1) Å, b = 6.126(1) Å, c = 5.636(1) Å, α = γ = 90°, β = 93.07(1)°

Picture: Rob Lavinsky, iRocks.com – CC BY-SA 3.0
https://commons.wikimedia.org/w/index.php?curid=10438776
Crystal structure (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
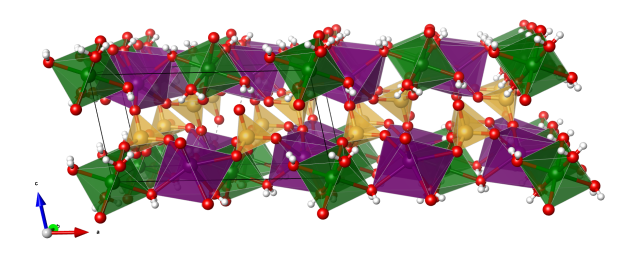
- CuO5Cl distorted octahedra (blue)
- CuO4Cl2 distorted octahedra (orange)
- Oxygen (red)
- Chlorine (green)
- Hydrogen (white)
For a 3D interactive version, see here:
Reference:
[1] Refinement of the Crystal Structure of Botallackite
F.C. Hawthorne
Mineralogical Magazine 1985, 49, 87-89.
DOI: 10.1180/minmag.1985.049.350.12
Cassiterite
- The name derives from the Greek kassiteros for tin
- Cassiterite has been the chief tin ore throughout ancient history and remains the most important source of tin today
- As often some of the tin atoms are substituted with iron, titanium, zirconium or tantalum, cassiterite, or to be precise, the smelting slag of SnO2, is in particular, also a source for tantalum
- Belongs to the Rutile mineral group
- Formula: SnO2
- Space group: P42/mnm (No. 136)
- Crystal system: tetragonal
- Crystal class: 4/mmm
- Lattice parameters: a = b = 4.7382(4) Å, c = 3.1871(1), α = β = γ = 90°

Picture: CarlesMillan – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
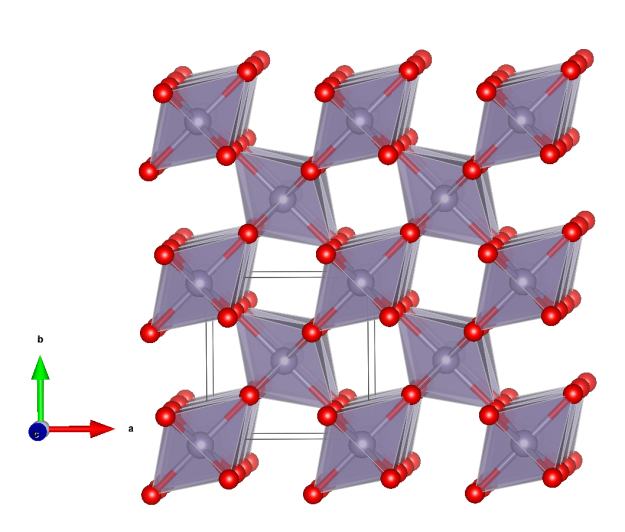
- SnO6 octahedra (slightly distorted) (purple)
- Oxygen (red)
For a 3D interactive version, see also here:
Ref.:
Structural Studies of Rutile-Type Metal Dioxides
A. A. Bolzan, C. Fong, B. J. Kennedy and C. J. Howard
Acta Cryst. B 1997, 53, 373-380
https://doi.org/10.1107/S0108768197001468
Celestine
- named from the Latin word caelestis meaning celestial, which in turn is derived from the Latin word caelum meaning sky or heaven because of its often soft blue color
- pure Celestine is colourless
- due to lattice defects in Celestine, colour centres are created which give the crystal its characteristic bluish colour
- these centers are often additionally stabilized by the presence of pottasium ions
- heating to over 200 °C “cures” these lattice defects and the mineral loses its color
- radiation with X-rays creates new or more lattice defects and the color returns or can be intensified.
- Formula: SrSO4
- Space group: Pnma (No. 62)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 8.360 Å, b = 5.352 Å, c = 6.858 Å, α = β = γ = 90°

Picture: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0
Crystal structure (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
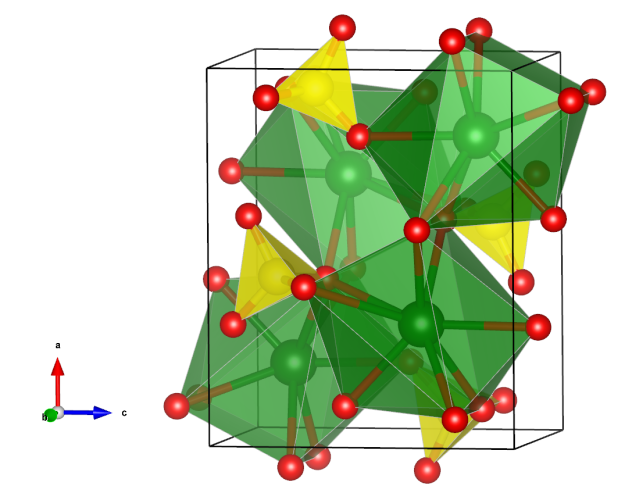
- SO4 tetrahedra (yellow)
- SrO8 polyhedra (green)
- Oxygen (red)
For a 3D interactive version, see here:
Chalcanthite
- Named after the greek words “chalkos” (= copper) and “anthos” (= flower)
- Formula: CuSO4 · 5 H2O
- Space group: P-1
- Crystal system: triclinic
- Crystal class: -1
- Lattice parameters: a = 6.141 Å, b = 10.736 Å, c = 5.986 Å, α = 82.27°, β = 107.43°, γ = 102.67°

Picture: Ra’ike (see also: de:Benutzer:Ra’ike) – CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=3948785
Crystal structure (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
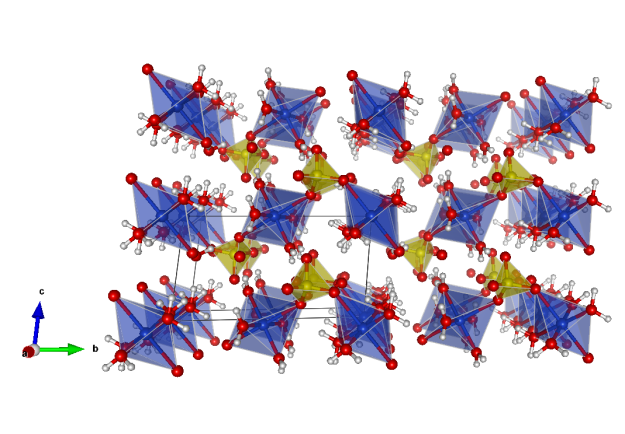
- CuO6 octahedra (blue)
- SO4 tetrahedra (yellow)
- Oxygen (red)
- Hydrogen (white)
For a 3D interactive version, see here:
Reference:
Neutron-diffraction studies of CuSO4 · 5 H2O and CuSO4 · 5 D2O
G.E. Bacon, D.H. Titterton
Zeitschrift für Kristallographie 1975, 141 (5-6), 330-341.
DOI: 10.1524/zkri.1975.141.5-6.330
Cinnabar
- probably named after the Persian word for dragon blood because of its characteristic red colour
- used since ancient times as a pigment
- Formula: HgS
- Space group: P3121 (No. 152) or P3221 (No. 154)
- Crystal system: trigonal
- Crystal class: 32
- Lattice parameters: a = b = 4.1347(6) Å, c = 9.4451(3) Å, α = β = 90°, γ = 120°

Picture: JJ Harrison (jjharrison89@facebook.com) – CC BY-SA 3.0
Crystal structure (click on the pictures to download the VESTA file):
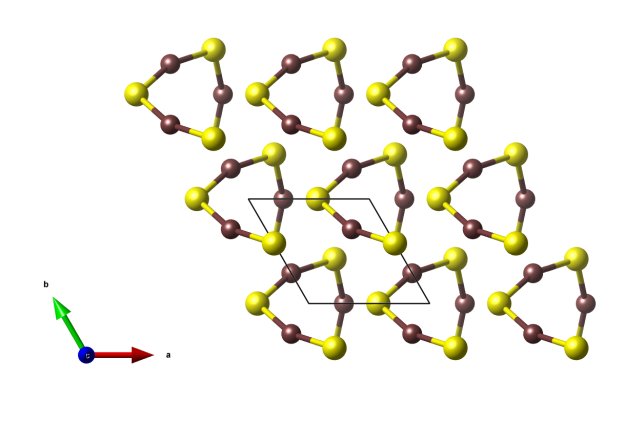
View along the c axis.
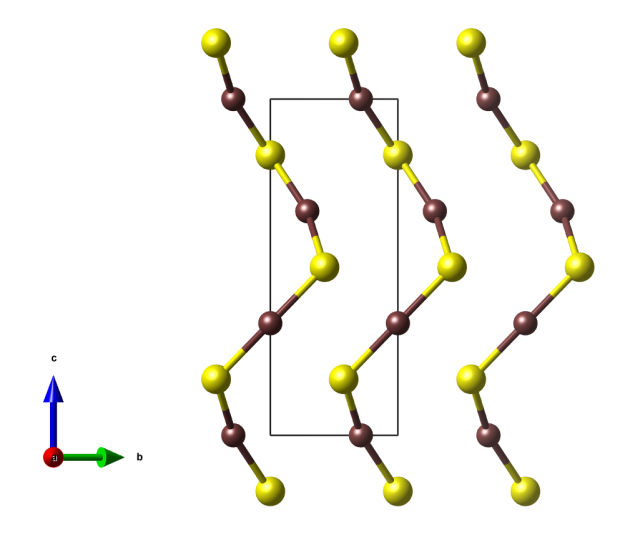
View along the a axis.
- the crystals structure is characteristic of chains with alternating sulfur and mercury atoms, which form helices with the helix axis running parallel to the c axis.
- These helices are also the reason why crystals of cinnarbar are optically active (see: A.M. Glazer, K. Stadnicka, “On the origin of optical activity in crystal structures”. J. Appl. Cryst. 19 (2), 1986, 108–122. doi:10.1107/S0021889886089823
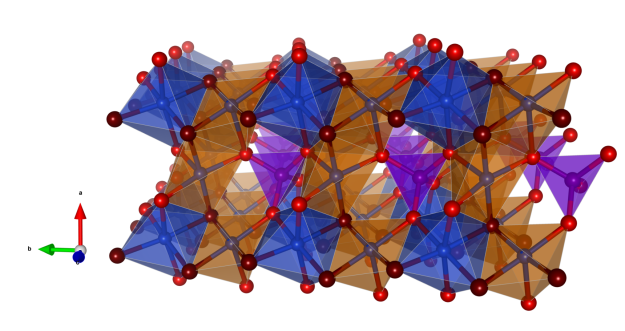
Clinoclase
- Named in 1830 by August Breithaupt from the Greek κλίυειυ = “to incline” and κλαυ = “to break,” in allusion to its oblique and perfect basal cleavage
- Formula: Cu3(AsO4)(OH)3
- Clinoclase is a polymorph of Gilmarite
- Space group: P21/c (No. 14)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 7.257(2) Å, b = 6.457(2) Å, c = 12.378(3) Å, α = 90°, β = 99.51(2)° γ = 90°

Picture: Yaiba Sakaguchi – http://www.mindat.org/photo-377299.html, public domain
https://commons.wikimedia.org/w/index.php?curid=27084499
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- AsO4 tetrahedra (purple)
- CuO6 slightly distorted octahedra (blue)
- CuO5 slightly distorted square pyramids (orange)
- Oxygen (red)
- Oxygen of OH groups (dark red)
For a 3D interactive version, see here:
Reference:
Clinoclase and the geometry of [5]-coordinate Cu2+ in minerals
R.K. Eby, F.C. Hawthorne
Acta Cryst. C 1990, 46, 2291-2294.
DOI: 10.1107/S0108270190004723
Crocoite
- The name crocoite comes from the Greek “krokos” = saffron, alluding to the saffron-orange color of its powder
- Formula: PbCrO4
- Space group: P21/n (No. 14)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 7.127 Å, b = 7.438 Å, c = 6.799 Å, α = 90°, β = 102.43°, γ = 90°

Picture: – http://www.mindat.org/photo-360746.html
(This image has been released to the public domain.)
Crystal structure (click on the picture to download the VESTA file):
View along the a axis.
Gray: Pb, red: O, orange: CrO4 tetrahedra
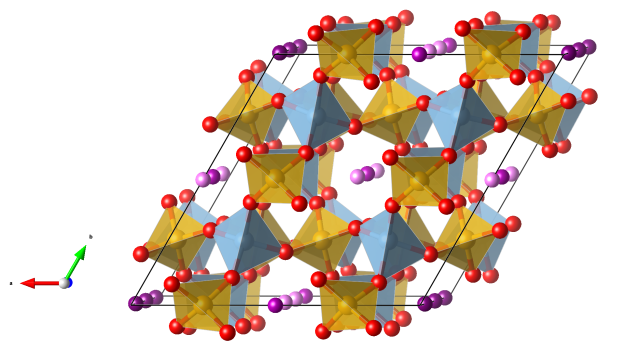
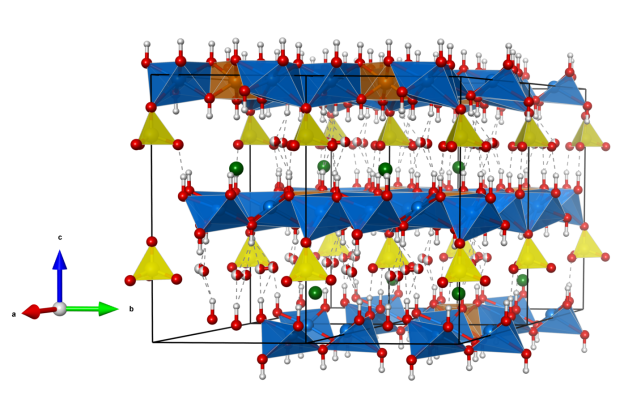
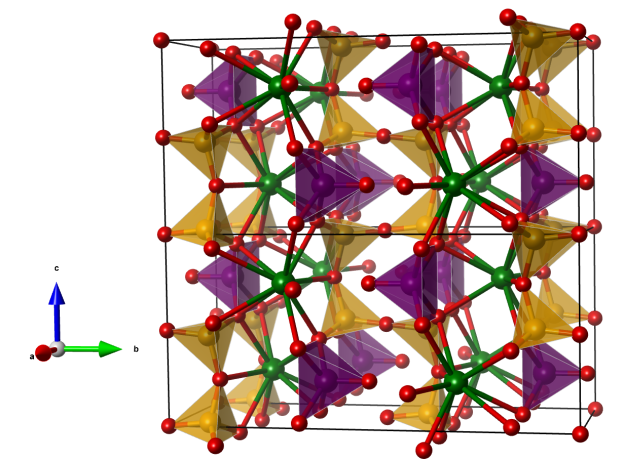
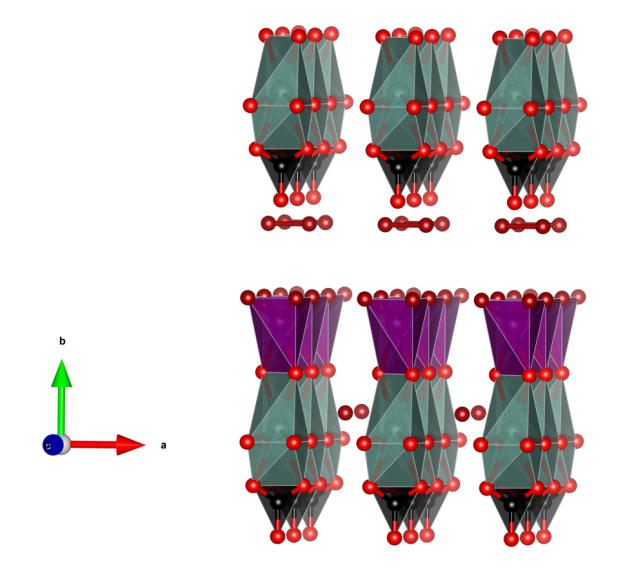
Despujolsite
- named in honor of Pierre Despujols (1888–1981), the founder of the Moroccan Geologic Survey (“Service de la carte géologique du Maroc”)
- The mineral was first observed in 1962 in manganese ore samples from Tachgagalt (Anti-Atlas, Morocco).
- Formula: Ca3Mn(SO4)2(OH)6 · 3 H2O
- Space group: P-62c
- Crystal system: trigonal
- Crystal class: -6m2
- Lattice parameters: a = b = 8.5405(5) Å, c = 10.8094(9) Å, α = β = 90°, γ = 120°

Picture: Rob Lavinsky, iRocks.com – CC BY-SA 3.0
Crystal structure (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- CaO8 polyhedra (blue)
- Mn(OH)6 octahedra (purple)
- SO4 tetrahedra (yellow)
- Oxygen (red)
- Hydrogen (white)
For a 3D interactive version, see here:
Refs:
[1] M.C. Barkley, H. Yang, S.H. Evans, R.T. Downs, M.J. Origlieri, Acta Cryst E 2011, 67, i47-i48.
DOI: 10.1107/S1600536811030911
Dioptase
- The name dioptase comes from the Greek words “dia” (= through) and “optos” (= visible), alluding to the visible cleavage planes inside the often highly transparent or translucent crystals
- Formula: CuSiO3 · H2O
- Space group: R-3 (No. 148)
- Crystal system: trigonal
- Crystal class: -3
- Lattice parameters: a = b = 14.566 Å, c = 7.778 Å, α = β = 90°, γ = 120°

Picture: Didier Descouens – CC BY-SA 3.0
Crystal structure (click on the picture to download the VESTA file):
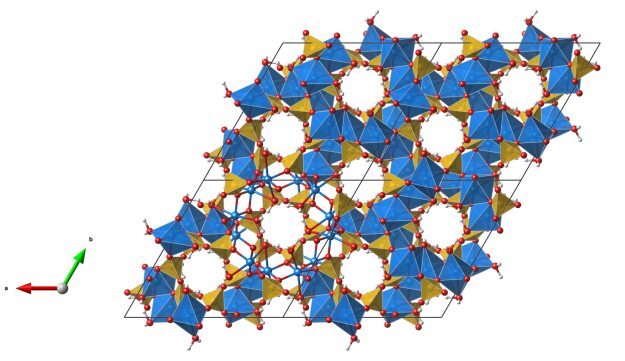
View along the c axis.
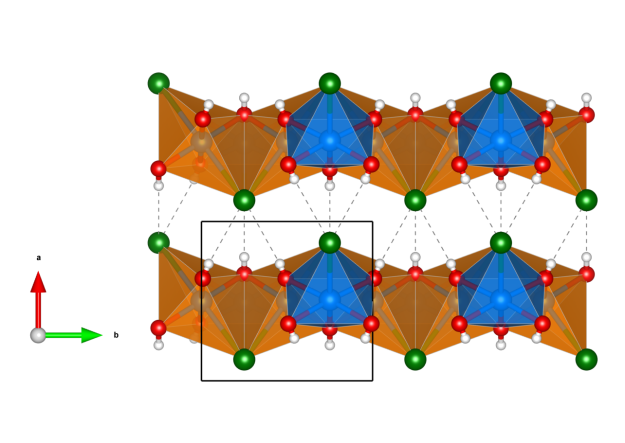
- six SiO4 tetrahedra (orange) are corner-connected to build a ring, so the mineral belongs to the cyclo silicates
- the copper atoms (blue) are octahedrally coordinated by six oxygen atoms (red), four of them are oxygens involved in SiO4 tetrahedra and they occupy the equatorial positions of the CuO6 octahedra and two of them are oxygen atoms of water molecules, which occupy the axial positions of the CuO6 octahedra
- the CuO6 octahedra are edge-connected and build a continuous 3D net
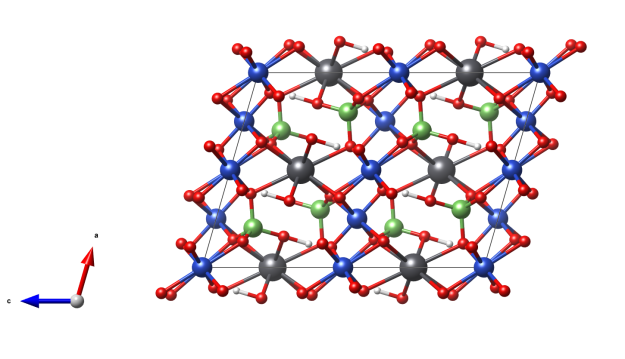
β-Eucryptite
- Named from the Greek for “well” and “concealed”, in reference to its occurrence as intimate intergrowths with the mineral albite
- It is the main component of the fameous glass-ceramic cooktops for stoves, known in the EU under the trademark Ceran® from Schott AG
- Formula: LiAlSiO4
- Space group: P6222 (No. 180)
- Crystal system: hexagonal
- Crystal class: 622
- Lattice parameters: a = b = 10.500 Å, c = 11.194 Å, α = β = 90°, γ = 120°
Crystal structure (click on the picture to download the VESTA file):
- SiO4 tetrahedra (orange)
- AlO4 tetrahedra (light blue)
- Li (purple/pink)
- Oxygen (red)
For a 3D interactive version, see here:
Freieslebenite
- Named after the mine commissioner of Saxony (Germany) Johann Carl Freiesleben
- Formula: AgPbSbS3
- Space group: P21/a (No. 14)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 7.518 Å, b = 12.809 Å, c = 5.940 Å, α = 90°, β = 92.25°, γ = 90°

Picture: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
View along the c axis.
- AgS3 (ditorted) trigonal-planar coordination environment (gray)
- SbS3 trigonal-pyramidal coordination environment (green)
- PbS6 distorted octahedra (orange)
Gilmarite
- Named in honour of Gilbert Mari (1944 – ), a mineralogist at the University of Nice-Sophia Antipolis, France.
- Formula: Cu3(AsO4)(OH)3
- Gilmarite is a polymorph of Clinoclase
- Space group: P1 (No. 1)
- Crystal system: triclinic
- Crystal class: 1
- Lattice parameters: a = 5.445(4) Å, b = 5.873(3) Å, c = 5.104(3) Å, α = 114.95(3)°, β = 93.05(5)° γ = 91.92(4)°
Crystal structure (click on the picture to download the VESTA file):
- CuO6 octahedra (blue)
- CuO5 square pyramids (orange)
- AsO4 tetrahedra (purple)
- Oxygen (red)
- Oxygen of OH groups (dark-red)
For a 3D interactive version, see here:
Reference:
Gilmarite, Cu3(AsO4)(OH)3, a new mineral: its description and crystal structure
H. Sarp, R. Cerny
European Journal of Mineralogy 1999, 11, 549-555.
Gottlobite
- Literally translated from German the meaning is “Thank God” or also “Praise God”; it is named after its type locality, the hill Gottlob (573 m) near Friedrichroda, Thuringia, Germany
- Known only since 1996
- Formula: CaMg(VO4,AsO4)(OH)
- Space group: P212121 (No. 19)
- Crystal system: orthorhombic
- Crystal class: 222
- Lattice parameters: a = 7.501 Å, b = 9.010 Å, c = 5.941 Å, α = β = γ = 90°

Picture: CC BY-SA 3.0 de – T – http://tw.strahlen.org/typloc/gottlobit.html
Crystal structure (click on the picture to download the VESTA file):
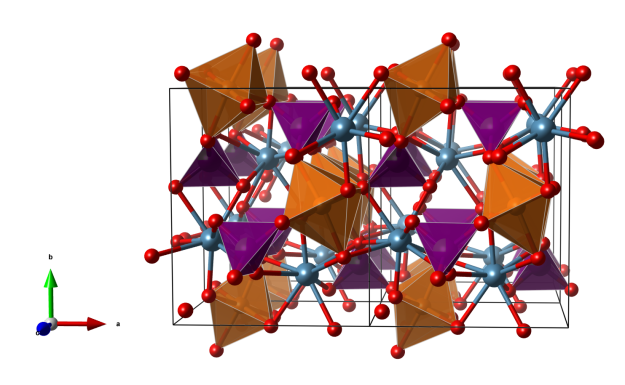
View along the c axis.
- MgO6 octahedra (orange)
- AsO4/VO4 tetrahedra (purple)
- Ca (blue)
- Oxygen (red)
For a 3D interactive version, see here:
Hercynite
- Named after the latin name Silva Hercynia of the Bohemian Forest, where this mineral was first found
- it is a ferro spinel
- Formula: FeAl2O4
- Space group: Fd-3m (No. 227)
- Crystal system: cubic
- Crystal class: m-3m
- Lattice parameters: a = b = c = 8.1458 Å, α = β = γ = 90°

| http://www.mindat.org/photo-73060.html
Crystal structure (click on the picture to download the VESTA file):
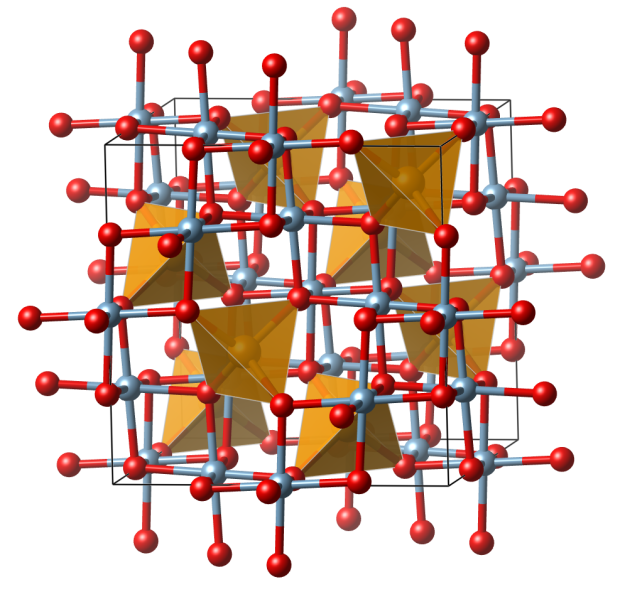
- FeO4 tetrahedra (orange)
- Al (blue), octahedral oxide coordination environment
- Oxygen (red)
For a 3D interactive version, see here:
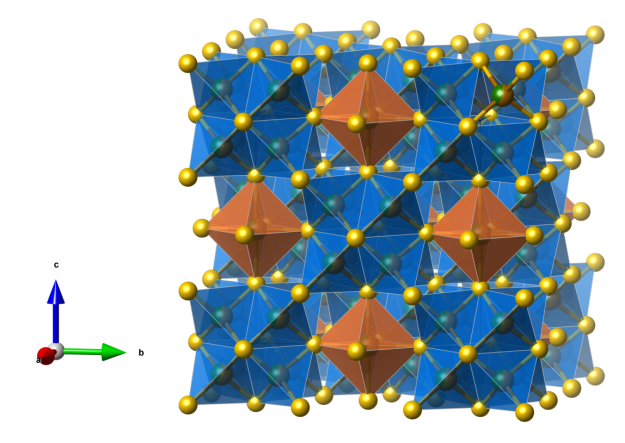
Hibbingite
- Named after its type locality, the city of Hibbing, which was built on the rich iron ore of the Mesabi Iron Range. At the edge of the town is the largest open-pit iron mine in the world.
- Hibbingite is isostructural with Atacamite [Cu2Cl(OH)3] and Kempite [Mn2Cl(OH)3]
- Formula: Fe2Cl(OH)3
- Space group: Pnma (No. 62)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 6.3373(2) Å, b = 6.9892(2) Å, c = 9.3457(3) Å, α = β = γ = 90°
Crystal structure (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
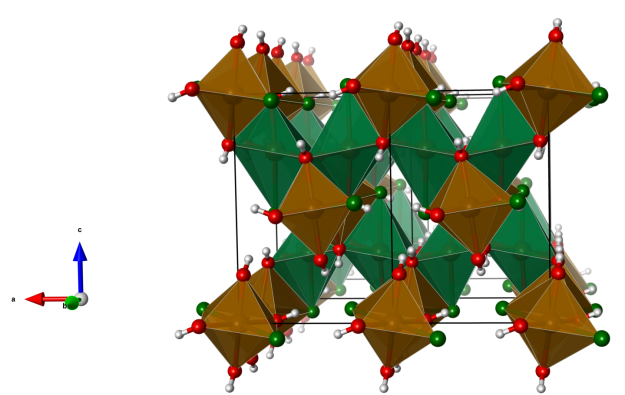
- FeO5Cl distorted octahedra (green)
- FeO4Cl2 distorted octahedra (brown)
- Oxygen (red)
- Chlorine (green)
- Hydrogen (white)
For a 3D interactive version, see here:
Reference:
N.V. Zubkova, I.V. Pekov, E.V. Sereda, V.O. Yapaskurt, D.Y. Pushcharovsky
Z. Kristallogr. 2019, 234 (6), 379-382.
DOI: 10.1515/zkri-2018-2124
Ilmenite
- Named after its type locality, the Ilmensky Mountais in the Southern Urals (Russia)
- Most important titanium mineral; it is predominantly mined for titanium dioxide production
- Formula: FeTiO3
- Space group: R-3 (No. 148)
- Crystal system: trigonal
- Crystal class: -3
- Lattice parameters: a = b = 5.0884 Å, c = 14.0855 Å, α = β = 90°, γ = 120°

Picture by Modris Baum (Public Domain)
Crystal structure (click on the picture to download the VESTA file):
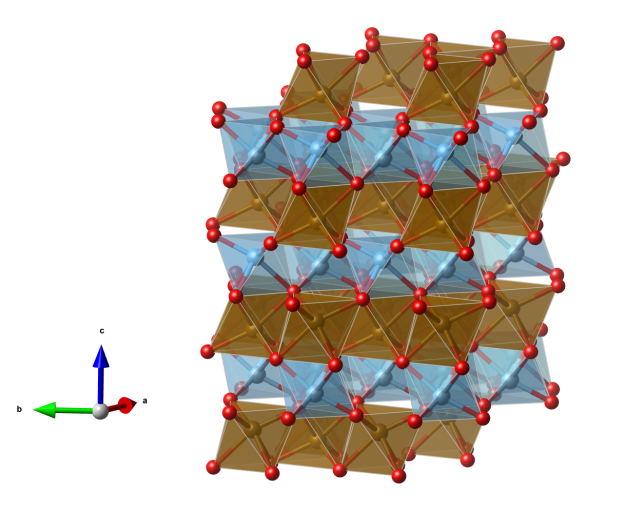
- FeO6 distorted octahedra (brown)
- TiO6 distorted octahedra (blue)
- Oxygen (red)
- The structure can be derived from the corundum (Al2O3) type: in corundum all cations are identical but in ilmenite Fe2+ and Ti4+ ions occupy alternating layers perpendicular to the c axis
- Within the alternating layers the FeO6 / TiO6 octahedra are edge-connected to give six-membered rings (the centre of the hexagons are unoccupied)
For a 3D interactive version, see here:
Jarosite
- Named after its type locality, Barranco del Jaroso in the Sierra Almagrera (near Los Lobos, Cuevas del Almanzora, Almería, Spain)
- In 2004 the Mars Exploration Rover – B found Jarosite on Mars. Because on Earth Jarosite is only formed in liquid water, this finding strongly indicates that Mars once possessed large amounts of liquid water.
- Formula: KFe3(OH)6(SO4)2
- Space group: R-3m (No. 166)
- Crystal system: trigonal
- Crystal class: -3m
- Lattice parameters: a = b = 7.304 Å, c = 17.268 Å, α = β = 90°, γ = 120°

Crystal structure (click on the picture to download the VESTA file):
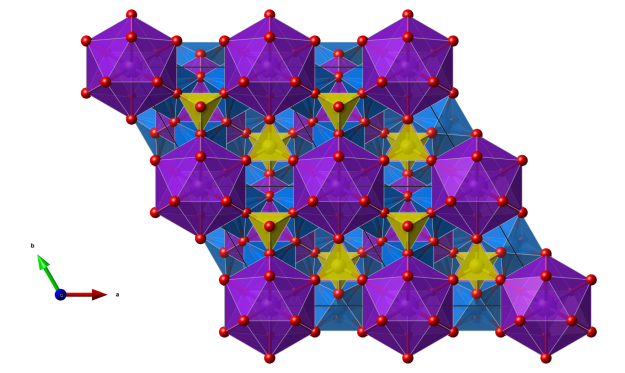
- KO12 only very slightly distorted icosahedra (purple)
- FeO6 octahedra (blue)
- SO4 tetrahedra (yellow)
- Oxygen (red)
For a 3D interactive version, see here:
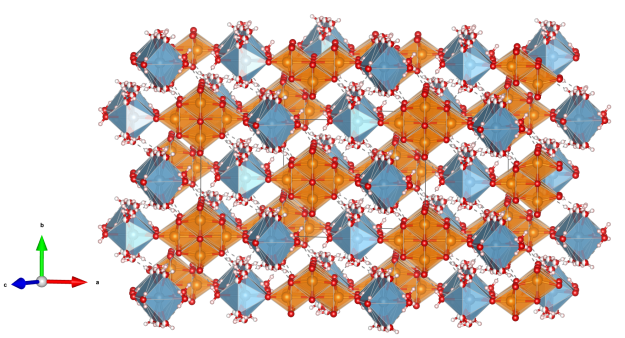
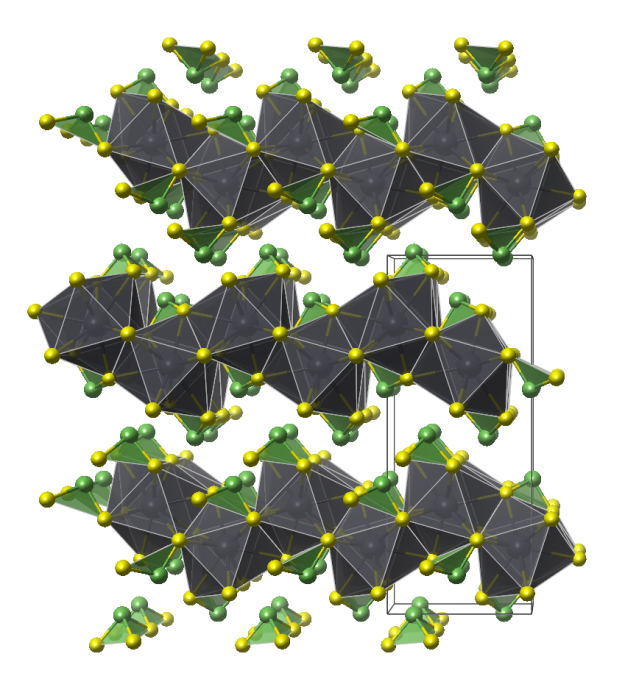
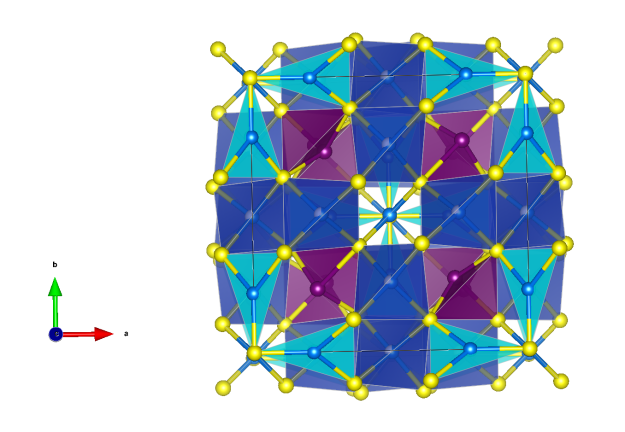
Kapellasite
- Named after Christo Kapellas (1938-2004), collector and mineral dealer of Kamariza, Lavrion, Greece
- Kapellasite is isostructural with Haydeeite [Cu3Mg(OH)6Cl2]
- Kapellasite is a metastable polymorph of Herbertsmithite
- Formula: Cu3Zn(OH)6Cl2
- Space group: P-3m1 (No. 164)
- Crystal system: trigonal
- Crystal class:- 3m
- Lattice parameters: a = b = 6.300(1) Å, c = 5.733(1) Å, α = β = 90°, γ = 120°
Crystal structure[1] (click on the pictures to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)

- CuO5Cl distorted octahedra (orange)
- CuO4Cl2 distorted octahedra (blue)
- Oxygen (red)
- Chlorine (green)
- Hydrogen (white)
For a 3D interactive version, see here:
Reference:
[1] W. Krause, H.-J. Bernhardt, R.S.W. Braithwaite, U. Kolitsch, R. Pritchard
Kapellasite, Cu3Zn(OH)6Cl2, a new mineral from Lavrion, Greece, and its crystal structure
Mineralogical Magazine, 2006, 70, 329-340
DOI: 10.1180/0026461067030336
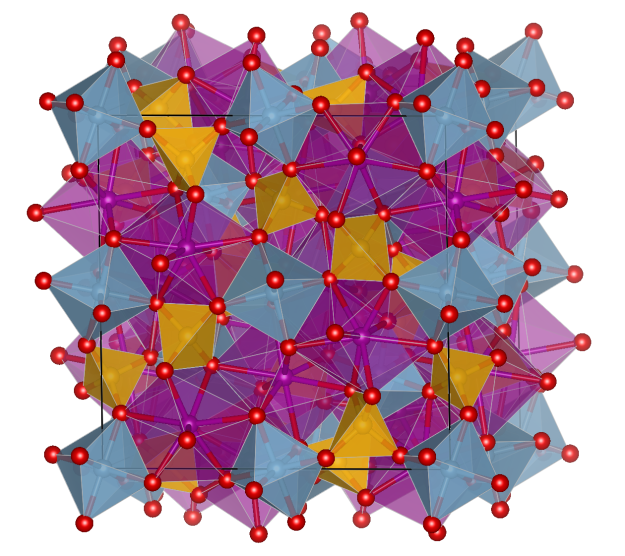
Keyite
- Named after the mineral dealer Charley Key, who discovered some tiny blue crystals of this mineral on a sample of green Adamite
- Formula: Cu3(Zn,Cd)4Cd2(AsO4)6· 2 H2O
- Space group: I2/a (No. 15)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 11.654 Å, b = 12.780 Å, c = 6.268 Å, α = γ = 90°, β = 99.11°

Picture: CC BY-SA 3.0 | Christian Rewitzer
Crystal structure (click on the picture to download the VESTA file):
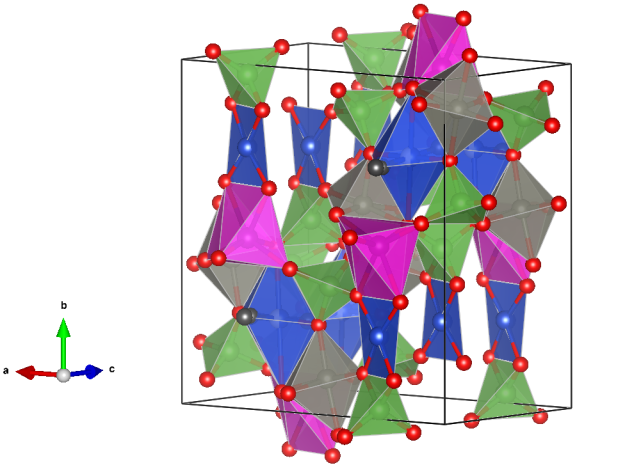
- CuO4 square-planar units (blue)
- CuO6 distorted octahedra (blue)
- CdO6 distorted trigonal prismatic units (pink)
- AsO4 distorted tetrahedra (green)
- Oxygen (red)
- Oxygen of water molecules (black)
For a 3D interactive version, see here:
Lavendulan
- The colour varies between turquoise and lavender, often also electric blue specimens can be found (see the picture below)
- Formula: NaCaCu5(AsO4)4Cl · 5 H2O
- Space group: P21/n (No. 14)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 10.011, b = 19.478 Å, c = 10.056 Å, α = 90° β = 90.37°, γ = 90°

Picture: CC BY-SA 3.0 | Christian Rewitzer
Crystal structure (click on the picture to download the VESTA file):
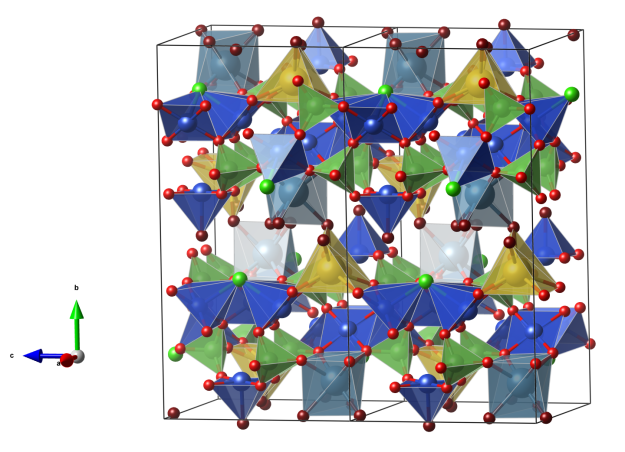
- CuO5 tetragonal pyramids (blue)
- CaO6 distorted octahedra (light blue)
- NaO6 distorted trigonal prismatic units (yellow)
- AsO4 tetrahedra (green)
- Oxygen (red)
- Oxygen of water molecules (dark red)
For a 3D interactive version, see here:
Mellite
- Also known as honeystone due to its often honey-yellow color
- Mellite is the aluminium salt of mellitic acid, i.e. benzene hexacarboxylic acid
- Formula: Al2C6(COO)6 · 16 H2O
- Space group: I41/acd (No. 142)
- Crystal system: tetragonal
- Crystal class: 4/mmm
- Lattice parameters: a = b = 15.553 Å, c = 23.110 Å, α = β = γ = 90°

Picture: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
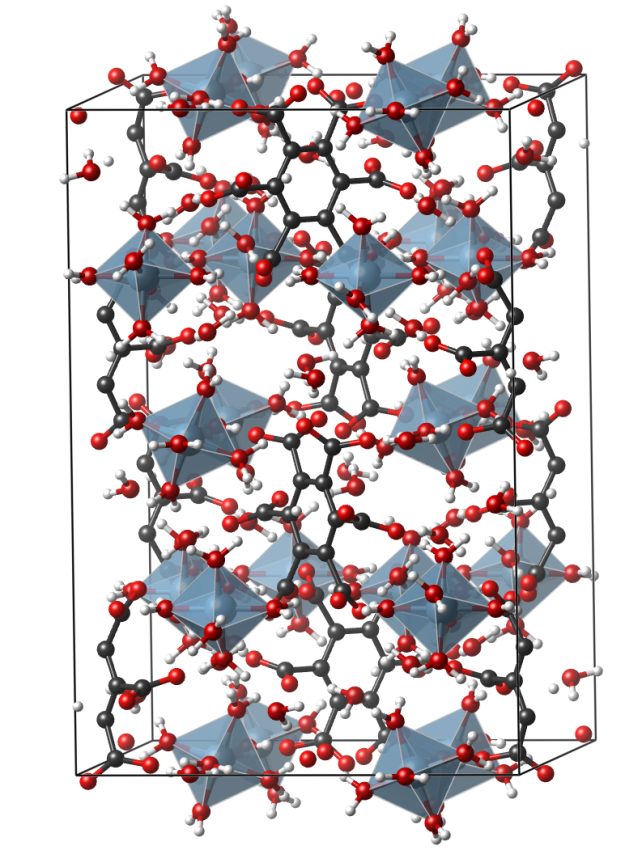
- AlO6 octahedra (blue)
- Oxygen (red)
- Carbon (black)
- Hydrogen (white)
For a 3D interactive version on sketchfab, see here:
Nadorite
- Named after its type locality Djebel Nador, Constantine, Algeria
- Formula: PbSbO2Cl
- Space group: Cmcm (No. 63)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 5.603 Å, b = 12.245 Å, c = 5.448 Å, α = β = γ = 90°

Picture: Christian Rewitzer – CC-BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- PbO4Cl4 square antiprisms (gray)
- SbO4 square pyramids (purple)
- Oxygen (red)
- Chlorine (green)
For a 3D interactive version on sketchfab, see here:
Osarizawaite
- Named after its type locality Osarizawa mine, Akita Prefecture, Japan
- Formula: PbCuAl2(SO4)2(OH)6
- Space group: R-3m (No. 166)
- Crystal system: trigonal
- Crystal class: -3m
- Lattice parameters: a = 7.075 Å, b = 7.075 Å, c = 17.248 Å, α = β = 90°, γ = 120°

Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- PbO12 (slightly distorted) icosahedra (gray)
- SO4 tetrahedra (yellow)
- Cu/AlO6 octahedra (blue)
- Oxygen (red)
- Hydrogen (white)
For a 3D interactive version on sketchfab, see here:
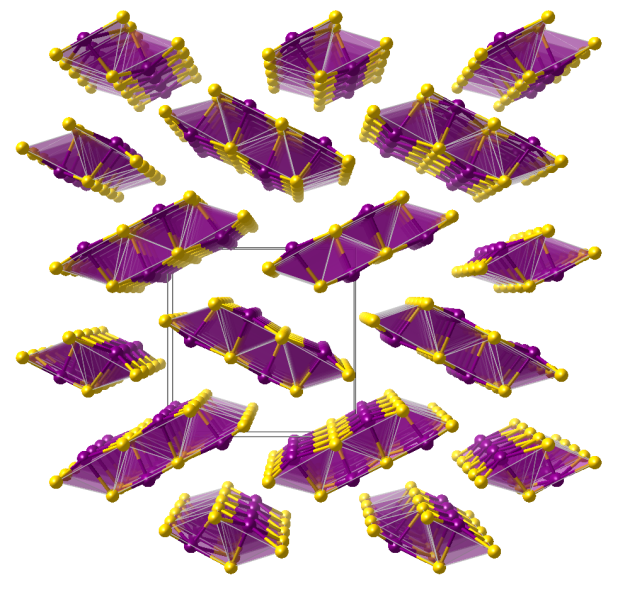
Pascoite
- Named after its type locality Pasco Province, Peru
- Formula: Ca3V10O28 · 17 H2O
- Space group: C2/m (No. 12)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 19.586 Å, b = 10.141 Å, c = 10.911 Å, α = γ = 90°, β = 120.815°

Picture: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- Clusters of Decavanadate anions [V10O28]6- (orange)
- CaO7 polyhedra (blue)
- Oxygen (red)
- Hydrogen (white)
For a 3D interactive version on sketchfab, see here:
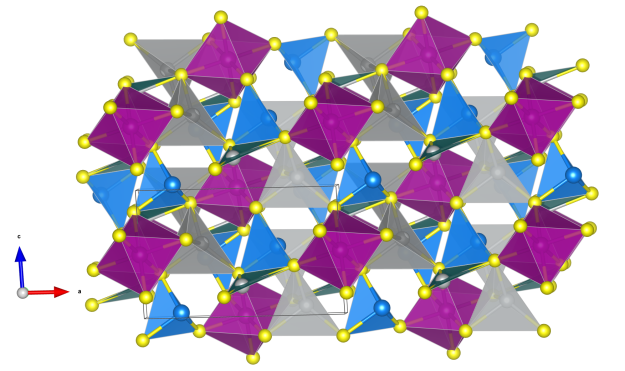
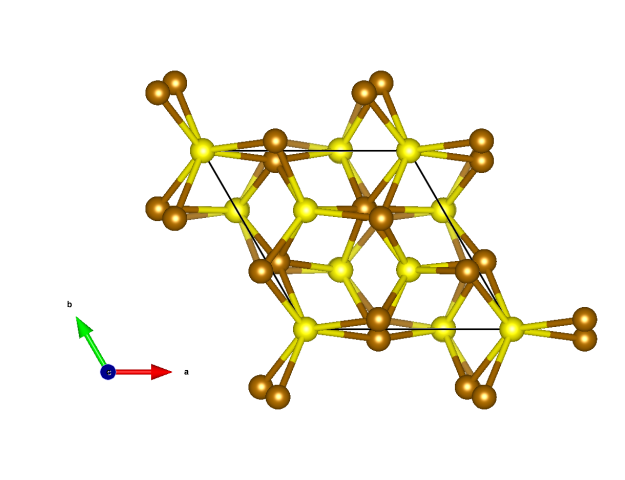
Pentlandite
- Named after the Irish natural historian, J. B. Pentland (1797-1873)
- Pentlandite is the most important nickel ore
- Formula: (Fe,Ni)9S8
- Space group: Fm-3m (No. 225)
- Crystal system: cubic
- Crystal class: m-3m
- Lattice parameters: a = b = c = 10.1075(1) Å, α = β = γ = 90°

Picture by: John Sobolewski (JSS) – http://www.mindat.org/photo-192760.html, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=9578696
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- For both metal cation positions there is a complete disorder between Ni and Fe.
- There are two distinct coordination environments; octahedrally coordinated metals at the center and all edge centers and tetrahedrally coordinated metals for the others.
- Eight tetrahedra each form edge-connected Fe/Ni8(µ-S)6S8 motifs, that means cubes of metal ions with six face-capping and eight terminal S atom. If we take now these cubes and octahedra as building blocks they form a NaCl-like structure.
- Fe/NiS4 tetrahedra (blue)
- Fe/NiS6 octahedra (orange)
- Fe (brown)
- Ni (green)
For a 3D interactive version on sketchfab, see here:
References:
[1] Tenailleau, C., Etschmann, B., Ibberson, R. M. & Pring, A.
A neutron powder diffraction study of Fe and Ni distributions in synthetic pentlandite and violarite using 60Ni isotope.
Am. Mineral. 91, 1442–1447 (2006)
[2] Stacey, T. E., Borg, C. K. H., Zavalij, P. J. & Rodriguez, E. E.
Magnetically stabilized Fe8(µ-S)6S8 clusters in Ba6Fe25S27.
Dalton Trans. 43, 14612–14624 (2014)
Portlandite
- Named portlandite because it is a common product of hydration of portland cement
- Formula: Ca(OH)2
- Space group: P-3m1 (No. 164)
- Crystal system: trigonal
- Crystal class: -3 2/m
- Lattice parameters: a = b = 3.5918 Å, c = 4.9063 Å, α = β = 90°, γ = 120°

Picture (public domain): SEM image of fractured hardened cement paste, showing plates of calcium hydroxide and needles of ettringite
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- CaO6 octahedra (blue)
- Oxygen (red)
- Hydrogen (white)
For a 3D interactive version on sketchfab, see here:
Rodalquilarite
- Named after its type locality, the Rodalquilar gold deposit, Almeria, Spain
- Formula: Fe2(TeO2OH)3(TeO3)Cl
- Space group: P-1 (No. 2)
- Crystal system: triclinic
- Crystal class: -1
- Lattice parameters: a = 5.103 Å, b = 6.653 Å, c = 9.012 Å, α = 73.40, β = 78.03° γ = 76.76°

Picture: Christian Rewitzer | CC BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- FeO6 octahedra (brown)
- TeO3 trigonal pyramids (blue)
- Oxygen (red)
- Hydrogen (white)
- Chlorine (green)
For a 3D interactive version on sketchfab, see here:
Samsonite
- Named after its type locality, the Samson Vein of Andreasberg silver mines, Harz Mountains, Germany.
- Formula: Ag4MnSb2S6
- Space group: P21/n (No. 14)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 10.3861 Å, b = 8.1108 Å, c = 6.6630 Å, α = γ = 90°, β = 92.639°

Picture: Christian Rewitzer | CC BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- MnO6 octahedra (purple)
- SbS3 trigonal pyramids (blue)
- AgS4 distorted tetrahedra (gray)
- AgS3 trigonal-planar coordination (green)
- Sulfur (yellow)
For a 3D interactive version on sketchfab, see here:
Sartorite
- Named after Wolfgang Sartorius von Waltershausen (1809 – 1876) Professor of Mineralogy, University of Göttingen, Germany. He was the first who described the mineral.
- Formula: PbAs2S4
- Space group: P21/n (No. 14)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 19.62 Å, b = 7.89 Å, c = 4.19 Å, α = γ = 90°, β = 90° (!)

Picture: Rob Lavinsky, iRocks.com – CC BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- PbS9 polyhedra (gray)
- AsS3 trigonal pyramids (green)
- Sulfur (yellow)
For a 3D interactive version on sketchfab, see here:
Schoenfliesite
- Named in 1971 by George T. Faust and Waldemar T. Schaller in honor of Arthur Moritz Schoenflies ( 17 April 1853 – 27 May 1928) Professor of Mathematics, University of Frankfurt. Schoenflies’ researches in group theory and topology resulted in his proof of the 230 space groups.
- Formula: MgSn(OH)6
- Space group: Pn-3 (No. 201)
- Crystal system: cubic
- Crystal class: m-3
- Lattice parameters: a = b = c = 7.7449(4) Å, α = β = γ = 90°
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- MgO6 octahedra (orange)
- SnO6 octahedra (blue)
- Hydrogen (white)
For a 3D interactive version on sketchfab, see here:
Reference:
Description of Schoenfliesite, MgSn(OH)6, and Roxbyite, Cu1.72S, from a 1375 BC Shipwreck, and Rietveld Neutron-diffraction Refinement of Synthetic Schoenfliesite, Wickmanite, MnSn(OH)6, and Burtite, CaSn(OH)6
L.C. Basciano, R.C. Peterson, P.L. Roeder
The Canadian Mineralogist 1998, 36, 1203-1210.
Spangolite
- Named in honour of Norman Spang (1841–1922), a mineral collector from Pennsylvania, USA; for details, see here.
- Spangolite is pyroelectric
- Formula: Cu6Al(SO4)(OH)12Cl · 3 H2O
- Space group: P31c (No. 159)
- Crystal system: trigonal
- Crystal class: 3m
- Lattice parameters: a = b = 8.254(4) Å, c = 14.354(8) Å, α = β = 90°, γ = 120°

Picture: Christian Rewitzer, CC BY-SA 3.0
https://commons.wikimedia.org/w/index.php?curid=14865917
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- CuO6 distorted octahedra (blue)
- AlO6 octahedra (orange)
- SO4 tetrahedra (yellow)
- Oxygen (red)
- Chlorine (green)
- Hydrogen (white)
For a 3D interactive version on sketchfab, see here:
References:
[1] Spangolite
H. A. Miers
Mineralogical Magazine 1894, 10, 273-277
DOI: 10.1180/minmag.1894.010.48.02
[2] The Crystal Structure of Spangolite, a Complex Copper Sulfate Sheet Mineral
F.C. Hawthorne, M. Kimata, R.K. Eby
American Mineralogist 1993, 78 (5-6), 649-652.
Spessartine
- Named after its type locality Spessart (Bavaria, Germany)
- Formula: Mn3Al2[SiO4]3
- Space group: Ia-3d (No. 230)
- Crystal system: cubic
- Crystal class: m-3m
- Lattice parameters: a = b = c = 11.621 Å, α = β = γ = 90°

Picture: Rob Lavinsky – iRocks.com | CC BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- MnO8 polyhedra (purple)
- AlO6 octahedra (blue)
- SiO4 tetrahedra (orange)
For a 3D interactive version on sketchfab, see here:
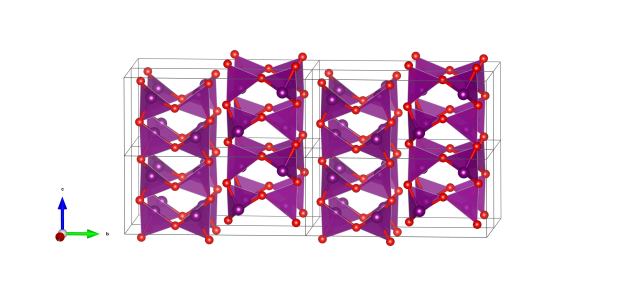
Stibnite
- Synonym: Antimonite
- The mineral has been known since ancient times and was used as a paste with fat as black make-up powder to color eyelids and eyebrows. In Arabic culture, dark eye rims are regarded as the ideal of beauty and at the same time as a magical repellent.
- Formula: Sb2S3
- Space group: Pnma (No. 62)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 11.3107 Å, b = 3.8363 Å, c = 11.2285 Å, α = β = γ = 90°

Picture: DerHexer, Wikimedia Commons, CC BY-SA 4.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,” J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- SbS5 tetragonal pyramids (purple)
For a 3D interactive version on sketchfab, see here:
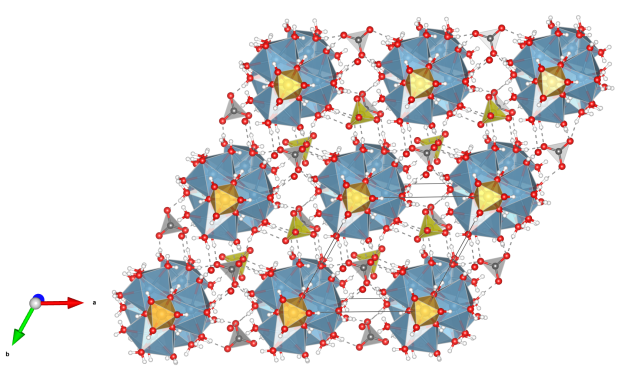
Strengite
- Named after Johann August Streng (1830-1897), German mineralogist, University of Giessen, Germany
- Formula: FePO4 · 2 H2O
- Space group: Pbca (No. 61)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 8.722 Å, b = 9.878 Å, c = 10.8117 Å, α = β = γ = 90°

Picture: Christian Rewitzer | CC BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- FeO6 octahedra (dark-red)
- PO4 tetrahedra (green-blue)
- Oxygen (red)
- Hydrogen (white)
For a 3D interactive version on sketchfab, see here:
Suzukiite
- Named in honor of Jun Suzuki, a professor of mineralogy and petrology, at the Hokkaido University, Sapporo (Japan).
- Suzukiite belongs to the inosilicates (chain silicates) and is polymorphic to Bavsiite, which is tetragonal.
- Formula: BaVSi2O7
- Space group: Cmcm (No. 63)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 5.3546(16), b = 15.249(5) Å, c = 7.094(2) Å, α = β = γ = 90°

Picture: D. Nishio-Hamane – CC BY-NC-SA 2.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- BaO10 polyhedra (not shown as polyhedra, Ba green)
- SiO4 tetrahedra (yellow)
- VO5 square pyramids (purple)
- Oxygen (red)
For a 3D interactive version on sketchfab, see here:
Reference:
Crystal Structure of Suzukiite from the Mogurazawa Mine, Gunma Prefecture, Japan,
M. Ito, S. Matsubara, K. Yokoyama, K. Momma, R. Miyawaki, I. Nakai, A. Kato
Journal of Mineralogical and Petrological Sciences 2014, 109, 222-227
DOI: 10.2465/jmps.140520
Tetrahedrite
- named after its common outer shape – a tetrahedron
- because of its relative high copper content it is also a (minor) ore of copper
- Formula: Cu12[S|(SbS3)4]
- Space group: I-43m (No. 217)
- Crystal system: cubic
- Crystal class: -43m
- Lattice parameters: a = b = c = 10.448 Å, α = β = γ = 90°

Picture: – http://www.mindat.org/photo-176605.html | CC BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- SbS3 pyramids (purple)
- CuS3 trigonal planar coordination (cyan)
- CuS4 tetrahedra (blue)
- Copper (blue)
- Sulfur (yellow)
- Antimony (purple)
For a 3D interactive version on sketchfab, see here:
Thaumasite
- first described in 1878 in Sweden and named from the Greek, “thaumazein”, to be surprised, in reference to its unusual composition with carbonate, sulfate and hydroxysilicate anions
- Formula: Ca3Si(OH)6(CO3)(SO4)
- Space group: P63 (No. 173)
- Crystal system: hexagonal
- Crystal class: 6
- Lattice parameters: a = b = 11.0538 Å, c = 10.4111 Å, α = β = 90°, γ = 120°

Picture: Rob Lavinsky| iRocks.com | CC BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- CaO8 polyhedra (blue)
- SiO6 octahedra (orange)
- SO4 tetrahedra (yellow)
- CO3 trigonalplanar coordination (gray)
- Oxygen (red)
- Hydrogen (white)
For a 3D interactive version on sketchfab, see here:
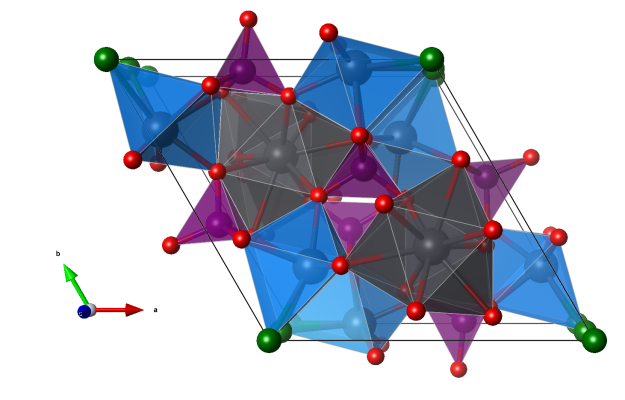
Thomasclarkite-Y
- Named in honor of Thomas Henry Clark (1893-1996), Professor of Geology, McGill University, Montreal, Quebec (Canada).
- Formula: Na(Y,REE)(HCO3)(OH)3 · 4 H2O (REE = Rare Earth Element, frequently Ce)
- Space group: P2 (No. 3)
- Crystal system: monoclinic
- Crystal class: 2
- Lattice parameters: a = 4.556(1) Å, b = 13.018(6) Å, c = 4.556(2) Å, α = 90°, β = 90.15°, γ = 90°

Picture: By Leon Hupperichs, CC BY-SA 3.0
https://commons.wikimedia.org/w/index.php?curid=14866495
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- NaO6 polyhedra (purple); in the original reference these polyhedra are described as bifurcated rhombic pyramids; I prefer something like “twisted triangular prism”, but any better suggestion is very welcome
- YO8 polyhedra (greenish); in the original reference described as bisdisphenoids
- (H)CO3 trigonal-planar coordination (black)
- Oxygen (red)
- Oxygen representing water molecules (dark red)
For a 3D interactive version on sketchfab, see here:
Reference:
Thomasclarkite (Y), a new sodium rare earth element bicarbonate mineral species from Mont Saint Hilaire, Quebec
J.D. Grice, R.A. Gault
Canadian Mineralogist 1998, 36 (5), 1293-1300.
Troilite
- Troilite occurs at only very few places on earth but is abundant on our moon and also on Mars. The most frequent occurrences on earth are within meteorites.
- Troilite was named after Domenico Troili who collected and examined samples of a meteorite that fell on earth in Albareto (near Parma) in 1766.
- Formula: FeS
- Troilite is the iron-rich endmember of the pyrrhotite group, the iron-deficient variant of FeS with the formula Fe(1-x)S (x = 0 to 0.2).
- Space group: P-62c (No. 190)
- Crystal system: hexagonal
- Crystal class: -6m2
- Lattice parameters: a = b = 5.962(2) Å, c = 11.750(3) Å, α = β = 90°, γ = 120°

A section of the meteorite Sikhote Alin that has Troilite inclusions.
Picture: André Knöfel, CC BY-SA 3.0 de
https://commons.wikimedia.org/w/index.php?curid=7803661
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
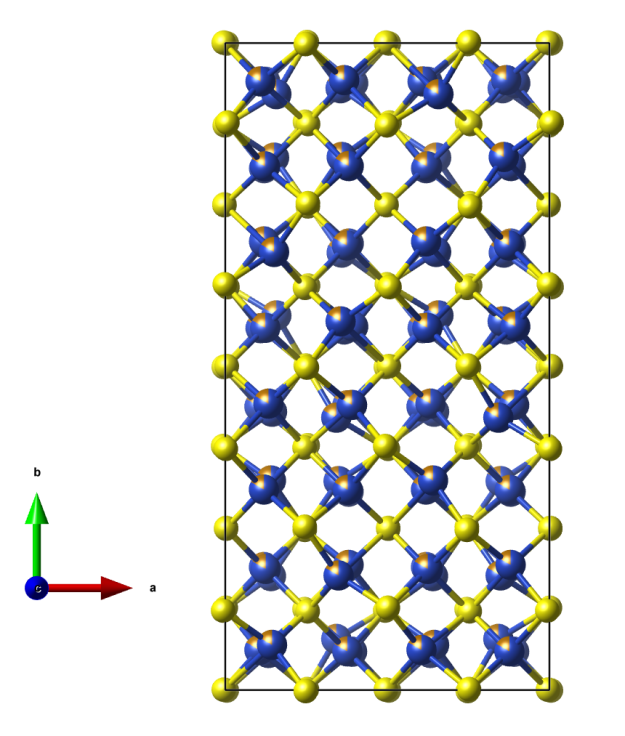
- Although it is a stoichiometric 1:1 compound the Fe (brown) and S (yellow) atoms do have different coordination environments:
- FeS6 (distorted) octahedra
- SFe6 (distorted) trigonal prisms
- This is analogous to NiAs; however, in Troilite the unit cell represents a superstructure of the simple NiAs-type structure (P63/mmc) based on small displacements of the Fe and S atoms from their ideal positions leading to an overall reduced symmetry (P-62c).
- The hexagonal close-packed sulfur framework remains essentially the same.
For a 3D interactive version on sketchfab, see here:
Reference:
Lunar Troilite: Crystallography
H. T. Evans Jr
Science 1970, 167, 621-623
DOI: 10.1126/science.167.3918.621
Vanadinite
- Named for its vanadium content
- Vanadinite is the most important source of vanadium and finds application as V2O5 in the famous contact process (production of sulfuric acid) and in alloys for chromium-vanadium steel (typically the content is below 0.2 %)
- Formula: Pb5(VO4)3Cl
- Space group: P63/m (No. 176)
- Crystal system: hexagonal
- Crystal class: 6/m
- Lattice parameters: a = b = 10.299(2) Å, c = 7.308(1) Å, α = β = 90°, γ = 120°

Picture: Didier Descouens – CC BY-SA 4.0
https://commons.wikimedia.org/w/index.php?curid=12436369
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- PbO9 irregular polyhedra (black)
- PbO7 capped trigonal prisms (blue)
- VO4 tetrahedra (purple)
- Oxygen (red)
- Chlorine (green)
For a 3D interactive version on sketchfab, see here:
Reference:
Crystal structure of vanadinite: refinement of anisotropic displacement parameters
F. Laufek et al.
J. Czech Geol. Soc. 2006, 51, 271-275
DOI: 10.3190/JCGS.999
Valentinite
- named in honour of Basilius Valentinus, a writer on alchemy. He is the supposed author of the first book to give a detailed description of antimony and its compounds.
- Formula: Sb2O3
- Space group: Pccn (No. 56)
- Crystal system: orthorhombic
- Crystal class: mmm
- Lattice parameters: a = 4.89960 Å, b = 12.4490 Å, c = 5.41030 Å, α = β = γ = 90°

Picture: Christian Rewitzer | CC BY-SA-3.0
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
- corner-connected SbO3 pyramids (purple)
- Oxygen (red)
For a 3D interactive version on sketchfab, see here:
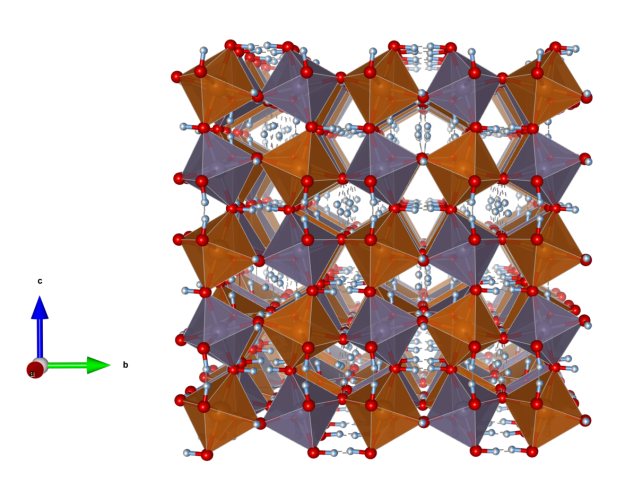
Vermiculite
- The mineral was first described in 1824 by Thomas H. Webb. Due to the property of the mineral to expand into worm-like formations when heated to 200 to 300 °C in the direction of the crystallographic c-axis, Webb named it after the Latin word “vermiculor” for “worm breeder”.
- On the occasion of the 195th anniversary of its discovery and due to its great importance as an industrial mineral, vermiculite was voted “Mineral of the Year” in Austria in 2019.
- Formula: Mg0,7(Mg,Fe,Al)6(Si,Al)8O20(OH)4 · 8 H2O
- Space group: C2/c (No. 15)
- Crystal system: monoclinic
- Crystal class: 2/m
- Lattice parameters: a = 5.349 Å, b = 9.255 Å, c = 28.89, α = γ = 90°, β = 97.12°

Picture: CC BY-SA 3.0 Leon Hupperichs
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
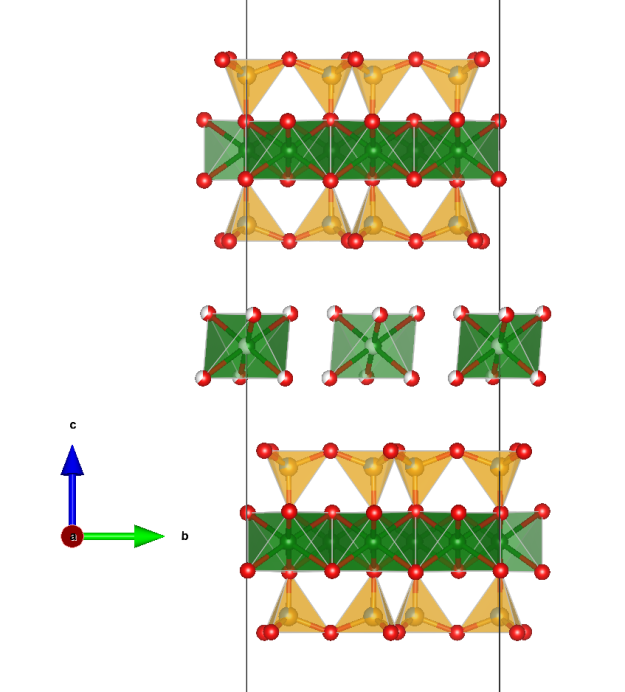
- Mg: green, Si: yellow, Al: blueish, O: red
- MgO6 octahedra (green), Si/AlO4 tetrahedra (yellow)
- Reference: Crystal structure of a two-layer Mg-vermiculite
H. Shirozu, S.W. Bailey
American Mineralogist (1966) 51 (7): 1124-1143.
http://www.minsocam.org/msa/collectors_corner/amtoc/toc1966.htm
For a 3D interactive version on sketchfab, see here:
Wulfenite
- Named in honour of Franz Xavier Wulfen (1728–1805), Austrian–German Jesuit, who wrote a
monograph on the lead ores of Bleiberg, Austria. - Wulfenite was voted “Mineral of the Year” in Austria in 2020.
- In its pure form wulfenite is colorless; the often yellow to red colour is most probably due to trace amounts of chromium substituting Mo.
- Formula: PbMoO4
- Space group: I41/a (No. 88)
- Crystal system: tetragonal
- Crystal class: 4/m
- Lattice parameters: a = b = 5.434 Å, c = 12.107 Å, α = β = γ = 90°

Picture: Didier Descouens – CC BY-SA 4.0
https://commons.wikimedia.org/w/index.php?curid=12426342
Crystal structure (click on the picture to download the VESTA file):
(K. Momma and F. Izumi, “VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data,”J. Appl. Crystallogr., 44, 1272-1276 (2011).)
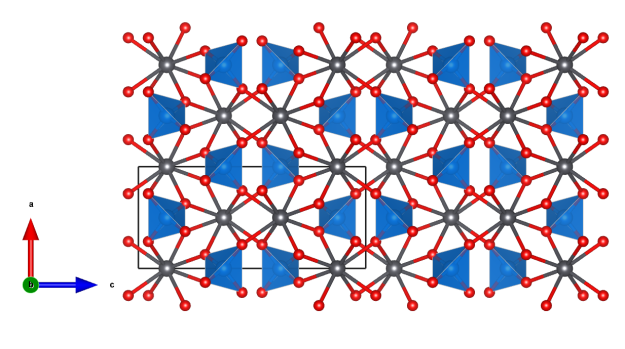
- PbO8 coordination environment
- MoO4 tetrahedra (blue)
- Pb (black)
- Oxygen (red)
For a 3D interactive version on sketchfab, see here:
Reference:
Natural wulfenite: structural refinement by single-crystal X-ray diffraction
C. Lugli, L. Medici, D. Saccardo,
Neues Jahrbuch fur Mineralogie, Monatshefte 1999, 6, 281-288

